Advisor: Dr. Martin Yarmush and Dr. Lawrence Williams
Office: BME 224
zrf6@rutgers.edu
Education
B.S., Chemical Engineering, University of Florida, 2014
B.S., Microbiology and Cell Science, University of Florida, 2014
PhD Candidate, Biomedical Engineering, Rutgers University
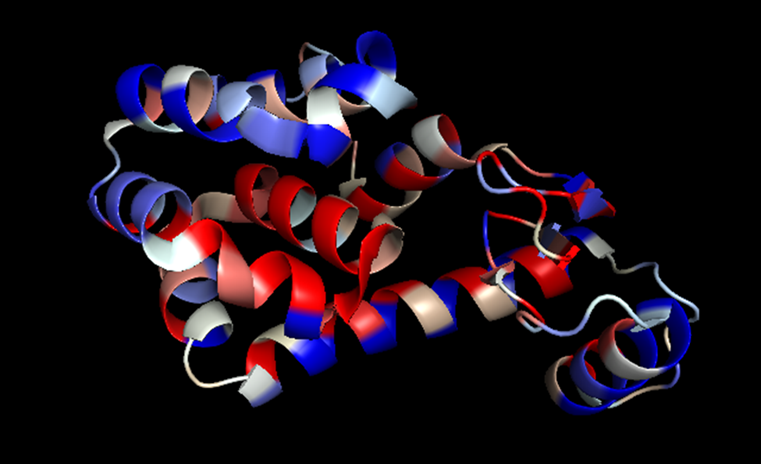 An example of one type of output from our computational protein energetics model. Here, the viral enzyme T4 lysozyme has been color-coded according to the interaction free energy contributions of each its amino acid residues. Red and salmon colored residues correspond to greater free energy contributions, with white, cyan, and dark blue corresponding to progressively lower contributions, respectively.
An example of one type of output from our computational protein energetics model. Here, the viral enzyme T4 lysozyme has been color-coded according to the interaction free energy contributions of each its amino acid residues. Red and salmon colored residues correspond to greater free energy contributions, with white, cyan, and dark blue corresponding to progressively lower contributions, respectively.Research Interests
Proteomics, immuno-oncology, biomarker discovery, bioassay development, molecular dynamics and computational modeling
Research Summary
Detecting cancer early in its progression is critical for reducing patient mortality and administering safer, more effective treatments. Recently research has shifted toward developing “liquid biopsy” techniques that test blood and other bodily fluids for the presence of certain disease-associated biomarkers; these tests may detect cancer earlier and would be less invasive than traditional screening methods. Autoantibodies specific to tumor antigens are one class of biomarker that appear very early and persist in many types of cancer, but tests to detect them are not widely used due to a low reported prevalence of individual autoantibodies in cancer patients. This is likely due to the inter- and intra-patient diversity inherent to the adaptive immune system, with even those patients that do produce autoantibodies exhibiting a heterogeneous, polyclonal response. Taking into account this heterogeneity, we believe that this low reported autoantibody frequency is due to the flawed molecular design immunoassays use to detect them. By using a new computational protein energetics model, we hypothesize that we are able to design superior, optimized peptide antigens that will capture and detect a larger proportion of the polyclonal autoantibodies in a cancer patient’s blood, significantly improving the sensitivity of this screening test. Additionally, we plan to develop an integrated microfluidic device that we hypothesize will enable the detection of lower concentrations of autoantibodies, further improving sensitivity and possibly allowing for earlier cancer detection. Our improved test will be applicable to a wide range of cancers, and may be useful for personalized medicine applications other than screening, such as monitoring for disease recurrence or determining which course of treatment to use.
Publications
Shrirao, A.B., Fritz, Z., Novik, E.M., Yarmush, G.M., Schloss, R.S., Zahn J.D., Yarmush, M.L. (2018). Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology, 6(1), 1-23, https://www.ncbi.nlm.nih.gov/pubmed/29682599
Krzyszczyk, P., Acevedo, A., Davidoff, E. J., Timmins, L. M., Marrero-Berrios, I., Patel, M., White, C., Lowe, C., Sherba, J. J., Hartmanshenn, C., O'Neill, K. M., Balter, M. L., Fritz, Z. R., Androulakis, I. P., Schloss, R. S., Yarmush, M. L. (2019). The growing role of precision and personalized medicine for cancer treatment. Technology, 6(3-4), 79-100. https://ncbi.nlm.nih.gov/pmc/articles/PMC6352312/
Representative Graduate Courses Taken
Biophysical Chemistry I
BioMEMS and Microfluidics
Kinetics, Thermodynamics, and Transport Phenomena
Advanced Technologies in Bioscience
Leadership & Outreach
Judge, North Jersey Regional Science Fair, 2019
Judge, New Jersey Junior Science and Humanities Symposium, 2018
President, Biomedical Engineering Student Society, 2016-2017