Advisor: Dr. Bonnie Firestein
Office: Nelson Biology Labs D413
aomelche@outlook.com
anton.om@icloud.com
Education
BA, Department of Psychology, CUNY Hunter College, 2014
PhD Candidate, Cell Biology & Neuroscience, Rutgers University
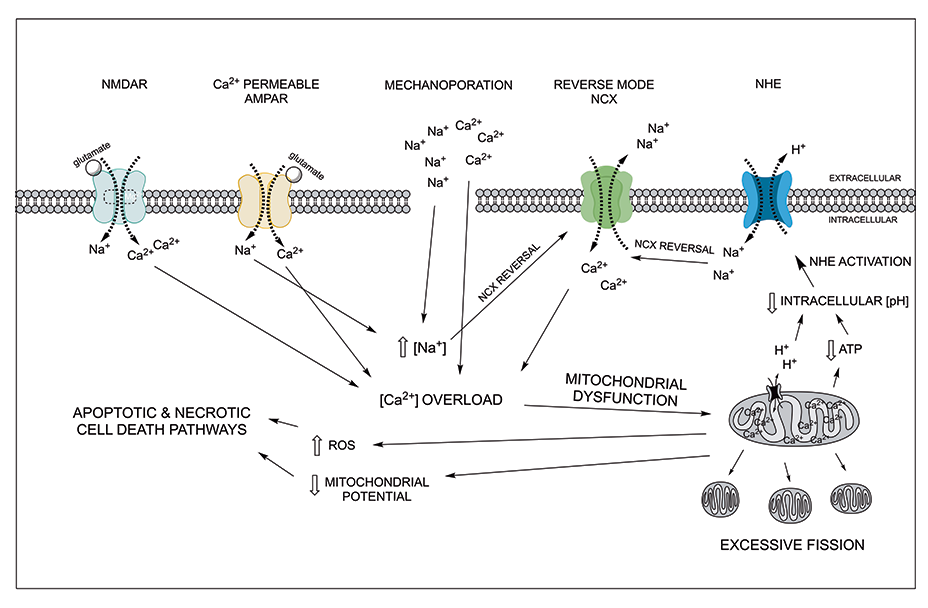 Schematic of secondary-phase TBI molecular cascade. Excess glutamate release following TBI induces aberrant neuronal depolarization and acidosis. Acidosis induces activation of the sodium hydrogen exchanger (NHE), which combined with ionotropic glutamate receptor activity and mechanoporation, leads to dysregulation of intracellular Na+ balance. The resulting increase in intracellular Na+ activates the reversal mode of NCX in neurons, which contributes significantly to excessive influx of Ca2+. Excessive Ca2+ influx through NCX, NMDA receptors, and Ca2+-permeable AMPA receptors leads to aberrant Ca2+ signaling, propagating mitochondrial dysfunction. Elevated intracellular Ca2+ is sequestered by mitochondria and leads to aberrant mitochondrial fission, increased reactive-oxidative species production, and a decrease in mitochondrial potential and ATP production. These cascades ultimately induce apoptotic and necrotic cell death. Pharmacologically targeting these processes may serve to mitigate secondary cell death following TBI.
Schematic of secondary-phase TBI molecular cascade. Excess glutamate release following TBI induces aberrant neuronal depolarization and acidosis. Acidosis induces activation of the sodium hydrogen exchanger (NHE), which combined with ionotropic glutamate receptor activity and mechanoporation, leads to dysregulation of intracellular Na+ balance. The resulting increase in intracellular Na+ activates the reversal mode of NCX in neurons, which contributes significantly to excessive influx of Ca2+. Excessive Ca2+ influx through NCX, NMDA receptors, and Ca2+-permeable AMPA receptors leads to aberrant Ca2+ signaling, propagating mitochondrial dysfunction. Elevated intracellular Ca2+ is sequestered by mitochondria and leads to aberrant mitochondrial fission, increased reactive-oxidative species production, and a decrease in mitochondrial potential and ATP production. These cascades ultimately induce apoptotic and necrotic cell death. Pharmacologically targeting these processes may serve to mitigate secondary cell death following TBI.Research Interests
Research concentration in cellular and molecular mechanisms behind brain and spinal cord trauma and discovery of therapeutics to promote recovery. Project focus on role of mitochondrial dysfunction in neuropathologies following neural injury.
Research Summary
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality for individuals under 45 years of age, and there is no treatment available for TBI-induced cell damage in the brain. Diffuse axonal injury (DAI), a common TBI pathology, results from severe axonal strain following primary insult. Following injury, the axonal cytoskeleton breaks down, slowing axonal recovery by interrupting axonal protein and organelle trafficking. Mitochondrial dysfunction plays a major role in the cellular damage that occurs during subsequent secondary injury. Excessive glutamate release by neurons and glia, mechanosensitive channel activation, and the disruption of the axonal plasma membrane as a result of injury result in an increase in calcium influx into neurons. Mitochondrial buffering of the excess calcium present in the intracellular space leads to increased production of reactive oxygen species, an excess in mitochondrial fission, decreased ATP production, and the release of pro-apoptotic factors. These processes ultimately lead to cell death and contribute to the symptoms of secondary injury in TBI. As such, understanding the etiology and progression of complex neuropathologies, such as TBI, can be extremely difficult. These studies generally use expensive in vivo animal models, the results of which can be tedious and difficult to analyze and which require a large number of animal subjects for histopathological tissue evaluation. Other studies use in vitro cell culture models, which can fail to recapitulate the structure, function, architecture, or physiology of neural tissues in vivo. The absence of in vitro models of complex circuit disorders that are 1) realistic, 2) reproducible, 3) amenable to high-content data acquisition, and 4) allow for precise control of injury and cell treatments has resulted in a major bottleneck that prevents vertical advancement, largely due to the difficulty in elucidating the cellular mechanisms of CNS disorders.
Awards & Honors
NIH T32 GM008339
Rolland S. Parker Award
The Livingston Welch Award
Undergraduate Research Initiative Fellowship
Publications
Shrirao AB, Kung FH, Omelchenko A, Schloss RS, Boustany, NN, Zahn JD, Firestein BL. (2017) Microfluidic platforms for the study of neuronal injury in vitro. Biotechnology and Bioengineering, 115, 815-830, doi:10.1002/bit.26519. https://www.ncbi.nlm.nih.gov/pubmed/29251352
Omelchenko A, Firestein BL. (2018) Lipids and phosphates at odds in synaptic depression. The Journal of Biological Chemistry, 293, 1568-1569, doi:10.1074/jbc.H117.813808. https://www.ncbi.nlm.nih.gov/pubmed/29414768
O’Neill KM, Donohue KE, Omelchenko A, Firestein BL. (2018) The 3′ UTRs of Brain-Derived Neurotrophic Factor Transcripts Differentially Regulate the Dendritic Arbor. Frontiers in Cellular Neuroscience, 12, 60, doi:10.3389/fncel.2018.00060. https://www.ncbi.nlm.nih.gov/pubmed/29563866
Svane KC, Asis EK, Omelchenko A, Kunnath AJ, Brzustowicz LM, Silverstein SM, Firestein BL. (2018) d-Serine administration affects nitric oxide synthase 1 adaptor protein and DISC1 expression in sex-specific manner. Molecular and Cellular Neuroscience, 89, 20-32, doi:10.1016/j.mcn.2018.03.011. https://www.ncbi.nlm.nih.gov/pubmed/29601869
Liang C, Carrel D, Omelchenko A, Kim H, Patel A, Fanget I, Firestein BL. (2018) Cortical Neuron Migration and Dendrite Morphology are Regulated by Carboxypeptidase E. Cerebral Cortex, bhy155-bhy155 doi:10.1093/cercor/bhy155. https://www.ncbi.nlm.nih.gov/pubmed/29982499
Ko J, Hemphill M, Yang Z, Sewell E, Na YJ, Sandsmark DK, Haber M, Fisher SA, Torre EA, Svane KC, Omelchenko A, Firestein BL, Diaz-Arrastia R, Kim J, Meaney DF, Issadore D. (2018) Diagnosis of traumatic brain injury using miRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip, 18, 3617-3630. doi:10.1039/c8lc00672e. https://www.ncbi.nlm.nih.gov/pubmed/30357245
Li H, Zaghloul N, Ahmed I, Omelchenko A, Firestein BL, Huang H, Collins L. (2019) Caffeine inhibits hypoxia-induced increase in HIF-1α and promotes neonatal neuronal survival. Experimental Neurology. 317, 66-77, doi:10.1016/j.expneurol.2019.01.014. https://www.ncbi.nlm.nih.gov/pubmed/30822423
Representative Graduate Courses Taken
Advanced Neurobiology
Nervous System and Behavior I & II
Bioengineering in the Biotechnology and Pharmaceutical Industries
Nano- and Micro-Engineered Biointerfaces
Leadership & Outreach
Neuroscience Day in Firestein Lab, Spring 2017